ATLANTA — Testing remains one of the key tools in controlling the spread of COVID-19. Doctors at Emory Healthcare and Children's Healthcare of Atlanta see promise in a newly approved at-home rapid test.
The Food and Drug Administration implemented an emergency use authorization for the Ellume at-home rapid test in December. According to the FDA, it's the first over-the-counter fully at-home diagnostic test approved by the agency. The U.S. Department of Defense struck a nearly $232 million deal with the Australian company to provide about 8 million tests in America.
Dr. Wilbur Lam, a Children's Healthcare of Atlanta and Emory pediatric hematologist and biomedical engineer, was part of the research team at Emory University that oversaw the testing and development of the new rapid antigen test. He said the groundbreaking research was funded by the National Institutes of Health, as part of the Rapid Acceleration of Diagnostics (RADx) program.
Lam said the tests are essential for combating the asymptomatic spread of COVID-19 and reopening.
"I think we're all trying to figure this out with as many stakeholders as you can imagine," Lam said. "Not just communities, but businesses, schools, there are a lot of people at the table."
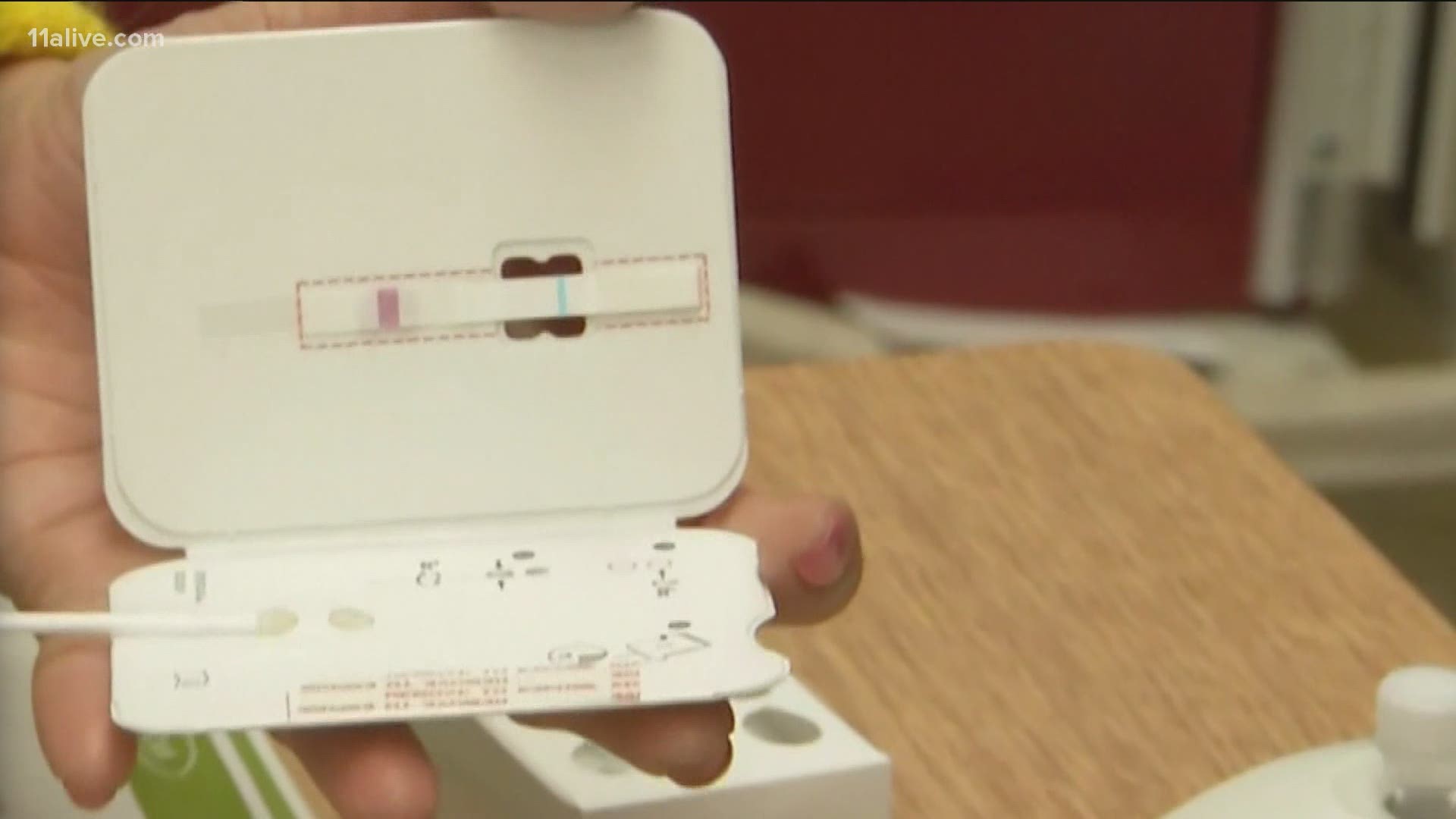
The test works by a self-nasal swab. The sample is run along a surface with reactive molecules, and it detects fragments of novel coronavirus proteins from a nasal swab sample. The test has been shown to work in people as young as 2-years-old according to the FDA.
The test uses a smartphone app that can send results to a lab and return them within 15-20 minutes of receiving a sample.
Lam said production was still ramping up, but he pointed to federal funding that would assist in the construction of Ellume's first manufacturing plant in the U.S. Once completed, the company estimated the plant would produce more than half a million tests per day.
In a U.S. clinical study, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms. In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples.
According to its website, the FDA has authorized more than 225 diagnostic kits for COVID-19 since the pandemic began, including Ellume's at-home rapid test.
Lam said two to three similar tests were undergoing research and could make it to market sometime this year. The physician said COVID-19 test measures are tested extensively. Healthcare professionals started researching the Ellume test in June, with virologists testing vials of coronavirus, pathologists using built-up banks of patient samples, and the conventional people willing to be enrolled in the study.
Lam said there is a potential for human error with the at-home rapid tests, and he recommends getting a follow-up PCR or antigen test if one gets a positive result.
Lam said Ellume was in talks with CVS to make the at-home rapid test widely available for retail. The suggested retail price was estimated to be about $30.
Lam said the timing of the release of the at-home rapid test could not have been better as vaccine rollouts continue across the country.
"The two coincide," Lam said. "As we know, vaccines are not perfect. Variants keep popping up, and we're working to find out if at-home rapid tests work with the variants as well."
Lam envisioned a slew of different tests to roll out in the next few months, as healthcare workers continue to figure out how frequently people should take the at-home test.