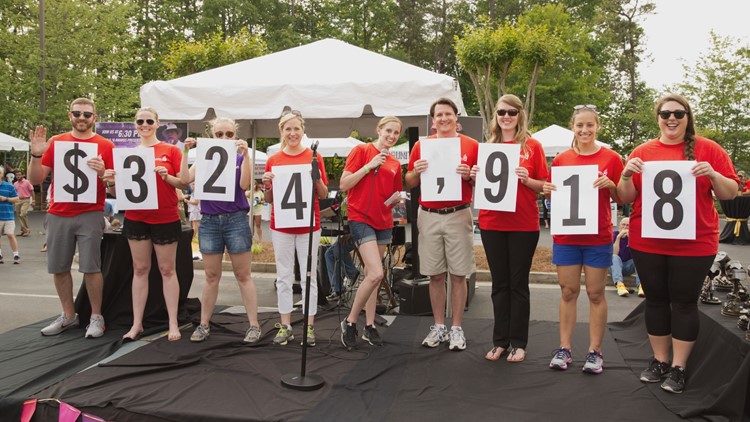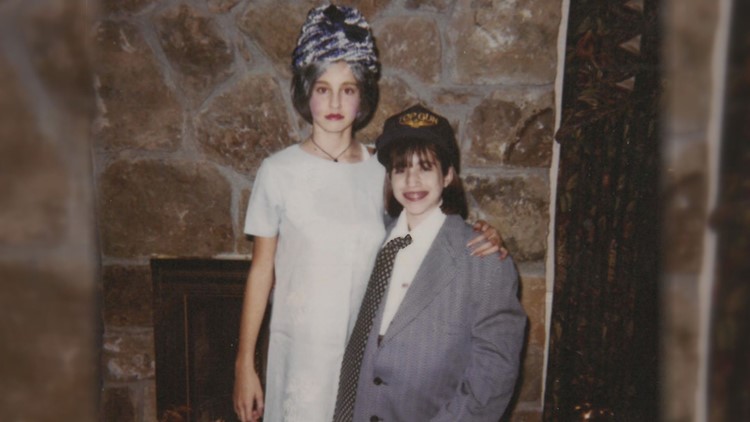Even she was too young to fully understand, Emily Choate Bridges has hoped for and worked towards this day.
“I hope everyone can understand the magnitude of this announcement,” she said.
The U.S. Food and Drug Administration approved a new drug that will change the lives of people with cystic fibrosis.
It's too late for her best friend, Leann Rittenbaum Ott. But this means it could help other families like hers.
Growing up, Emily watched Leann fight through the disease that attacked her lungs. The average age of survival at that time was just 13.
“Leann never felt sorry for herself,” Emily said. “She was a big personality in a small body and had such a joy and strength.”
The Choate family made it their life’s work to help fund research for a cure - for Leann and thousands like her. Their company in Atlanta, Choate Construction, has helped raise millions of dollars for research.
Every year they host a Cars & Q fundraiser with classic cars and barbecue. It was an event that Emily and Leann would go to together. They did everything they could - together.
Leann was 33-years-old when she passed away on Nov. 1, 2018.
Photos: Leann and Emily
Soon after, there came word of a possible medical breakthrough.
Trials were happening - including at Emory and Children’s Healthcare of Atlanta - with a combination of drugs that could help 90 percent of people with the disease that restricts their lungs.
“Many of our patients with low lung function will feel like they are struggling to breathe and shortness of breath, like they are breathing through a straw," explained Dr. Rachel Linnemann.
Linnemann is a pediatric pulmonologist at Children’s Healthcare of Atlanta and an assistant professor at Emory University School of Medicine.
This week came an announcement that the triple-combination drug is the game changer they hoped for. The U.S. Food and Drug Administration approved the use of the triple-combination modulator - which includes elexacaftor, tezacaftor, vacaftor - or Trikafta.
People with cystic fibrosis ages 12 and older, who have at least one copy of the F508del mutation, can use the drug. It means 90 percent of people with CF will have a highly-effective therapy for the underlying cause of their disease.
“It feels like a new miracle drug," Linnemann said. “This is the fruit of our labor.”
Bridges thanked everyone for all the years of support and generosity. There are events and families all over the country who have been working together towards this outcome.
“While we ran out of time with Leann, it is her life that will propel us forward," she said.
Emily plans to keep working to raise money for research to help the other 10 percent of people living with CF with even better treatments for all.
It’s what Leann and Emily hoped for and worked for, together.
“It’s not a cure, but it is dang close,” Emily said.
“As we celebrate today, we will not lose sight of the many individuals in our community who are still waiting for a breakthrough that will treat their mutations,” said Michael P. Boyle, M.D., the senior vice president of therapeutics development.
“We will not be done until every person with CF has a treatment for the underlying cause of their disease and, one day, a cure.”
---
Get more information from the Cystic Fibrosis Foundation.
MORE HEALTH-RELATED HEADLINES: