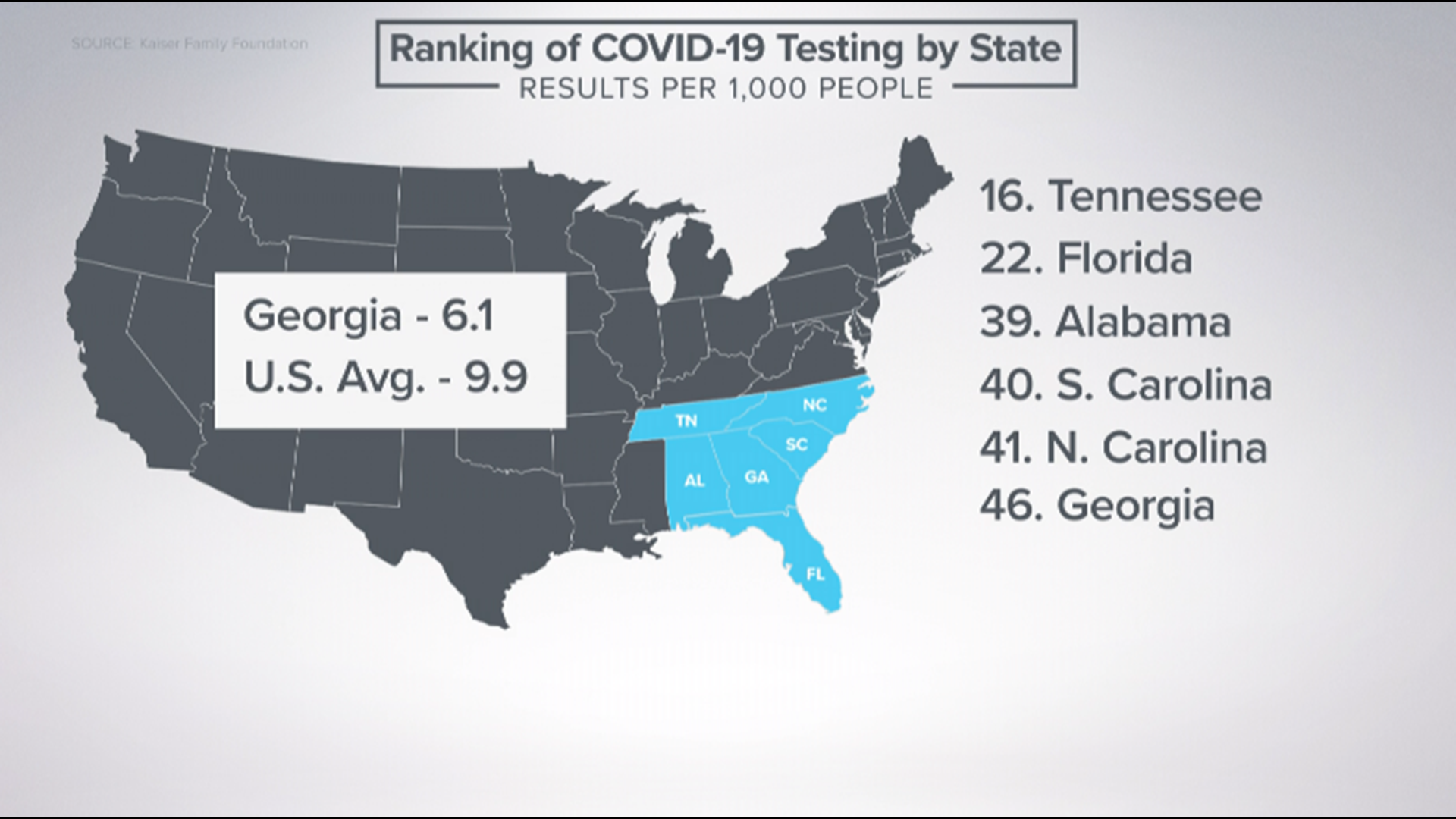
ATLANTA — According to an investigative report published by the Washington Post, a failure by the federal Centers for Disease Control and Prevention to quickly produce a test kit for detecting COVID-19 came by a "glaring scientific breakdown" at the CDC's facilities in Atlanta.
The original story, posted online early Saturday afternoon, said the CDC facilities "violated sound manufacturing practices" which ended up in contamination of one of the three test components used in the detection process.
In a statement, CDC spokesperson Benjamin Haynes told 11Alive on Saturday afternoon that the issue was being investigated by the Department of Health and Human Services.
Haynes said that when more information is available, they would provide details regarding the results of the investigation.
"As is well known and reported, some states had trouble validating the initial CDC diagnostic test," Haynes told 11Alive. "As of March 23, more than 90 state and local public health labs in 50 states, the District of Columbia, Guam and Puerto Rico verified they are successfully using COVID-19 diagnostic tests."
Haynes provided 11Alive with the questions that they had answered
The CDC had been asked what caused the third, pan coronavirus (N-3) component of the CDC's diagnostic test kit to fail. More specifically, "Was design a factor? Or, was contamination during manufacturing a factor?"
"This issue is being investigated by HHS," the statement from Haynes reiterated. "The issue with the N3 component of CDC's diagnostic test for COVID-19 may be the result of a design and/or manufacturing issue or possible contamination."
The report went on to ask why that third component was designed into the test kit -- and why the CDC delayed the decision to ultimately authorize labs to test for COVID-19 without the third component.
"The N3 component of CDC's diagnostic test for COVID-19 was intended to detect the presence of general coronavirus," Haynes said. "The first step after receiving a test kit is that states must verify that the test works. When some of the state public health laboratories were not able to validate the test in their laboratories, CDC immediately put a hold on the distribution of all test kits."
Haynes went on to point out that once the CDC identified the component that was not working correctly, their scientists worked to validate the test's effectiveness without it and to remanufacture the kits.
"We also worked to obtain FDA permission to drop the problematic component from the Emergency Use Authorization before rolling the test kit back out to the states to get testing up and running again.," he said.
11Alive is focusing our news coverage on the facts and not the fear around the virus. We want to keep you informed about the latest developments while ensuring that we deliver confirmed, factual information.
We will track the most important coronavirus elements relating to Georgia on this page. Refresh often for new information.
MORE CORONAVIRUS HEADLINES |